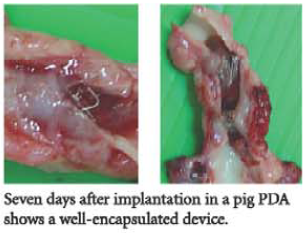
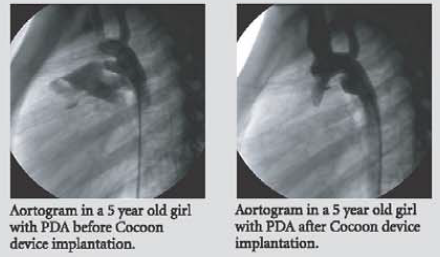
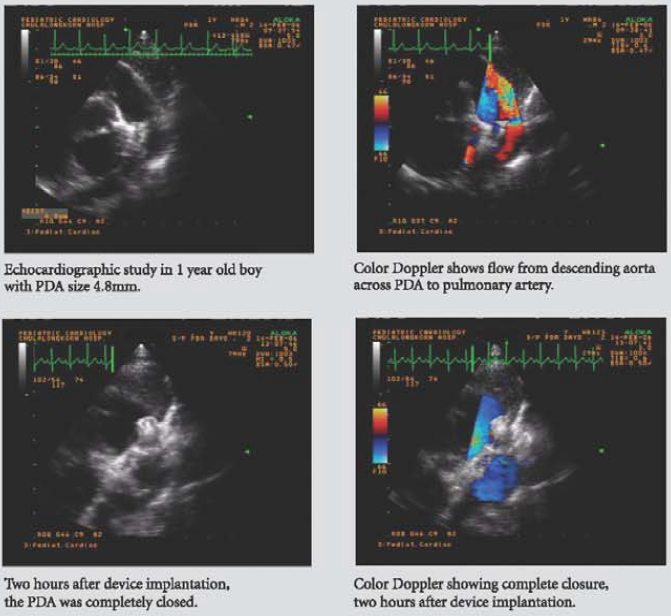
The Cocoon Duct Occluder is a percutaneous transcatheter occlusion device used in closure of patent ductus arteriosis. It is made from Nitinol wires coated with platinum using NanoFusion technology.
An extended retention portion on the aortic side provides for proper positioning of the device in the ductus arteriosus. The device is filled with polyester fabric to assist thrombogenicity.
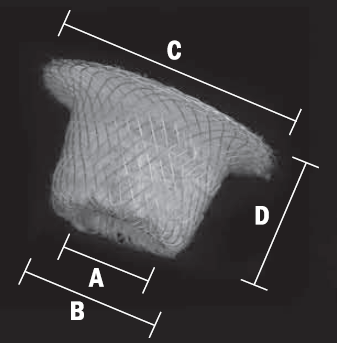
| Catalog Number |
Device Diameter at Plmonary Artery |
Device Diameter at Descending Aorta |
Retention Diameter |
Device Length |
Recommended Sheath size |
|---|---|---|---|---|---|
| COP0406 | 4 mm | 6 mm | 10 mm | 7 mm | 6-7 French |
| COP0608 | 6 mm | 8 mm | 12 mm | 7 mm | 6-7 French |
| COP0810 | 8 mm | 10 mm | 16 mm | 8 mm | 7-8 French |
| COP1012 | 10 mm | 12 mm | 18 mm | 8 mm | 7-8 French |
| COP1214 | 12 mm | 14 mm | 20 mm | 8 mm | 9-10 French |
| COP1416 | 14 mm | 16 mm | 22 mm | 8 mm | 9-10 French |
| COP1618 | 16 mm | 18 mm | 24 mm | 8 mm | 9-10 French |
| COP1820 | 18 mm | 20 mm | 26 mm | 8 mm | 10 French |