Commited to conducting clinical trials, Vascular Concepts has completed various trials and registries with Pronova in 2078 patients both in India and abroad. Following are the details of the clinical trials:
Don't have Adobe Acrobat? Click Here
ProNOVA
Features
Features |
Technical Data |
Ordering Info |
Abstracts |
Studies |
Images |
Download Brochure (pdf)

Sirolimus Eluting Stent
- Only stent incorporating variable strut thickness (VST) and variable strut design (VSD)
- Thin 60m S-Shaped articulations in mid portion ensures flexibility and avoids flare up of the struts
- Straight mid portion 90m articulations gives High Radial Strength
- Thicker metal coverage at the ends (120) to ensure more drug on the edges- Reduces edge restenosis.
Polymer
- Propriety blend of bio-compatible, bio-stable polymer
- Non-Inflammatory and Non-thrombogenic Polymer
- Polymer screened as per ISO 10993 Bio-compatibility tests
- Hemocompatibility test performed at NAMSA (North American Scientific Associates)
Drug
- Sirolimus most efficacious drug for stent delivery is used on stent
- Drug is cytostatic, acts on specific targets in the cellular proliferation cycle
- Sirolimus has the largest number of clinical trials and longest duration of follow-up as a drug on stent to reduce restenosis
Patented Vibrational Technology
- Patented Vibrational Technology ensures effective coating
- Drug loaded on the polymer matrix is securely attached to apre-functional stainless steel surface
Quality Control
- Stents and balloon catheters are manufacted in Germany
- Drug is exclusively licensed and procured from USA
- Drug is manufactured in a US FDA approved site under GMP conditions
- The final product is "finished" in an ISO 13485 facility
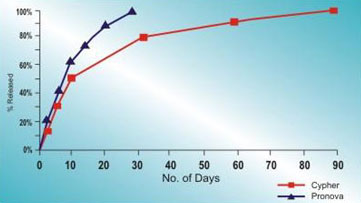
Release Kinetics
- About 50% of the drug is realeased within the first 10 days
- After 30 days, less than 25% of the drug remains on the stent surface
- Slow sustained realeased from the polymer matrix on the stent
Pronova comparative drug release kinetics